What did scientists discover?
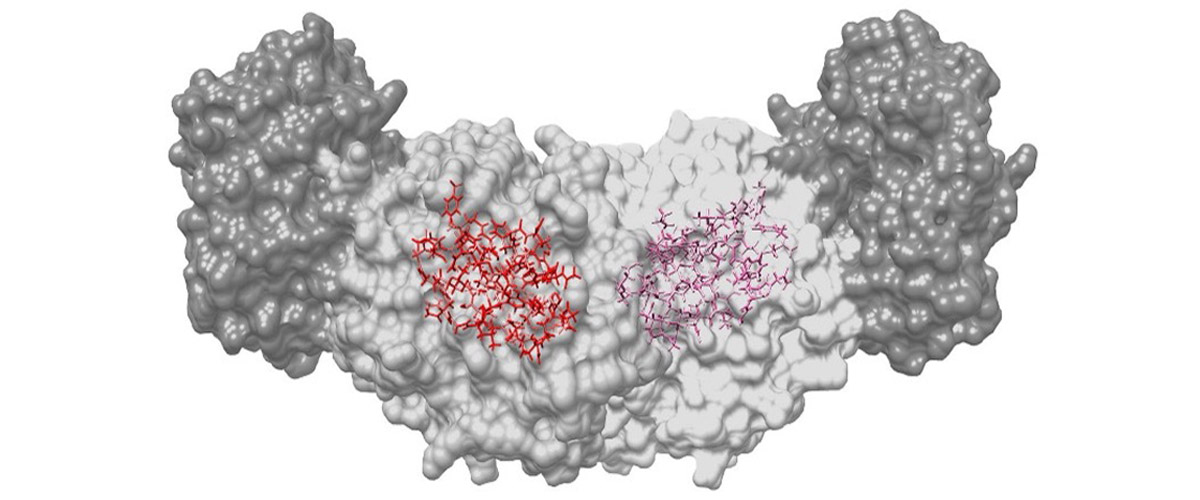
Hydrogen makes up half of the atoms in a protein. Without seeing where they are, creating a true picture of the chemical interactions, and how the molecules fit together, is impossible. UC Riverside and MagLab scientists have developed a new integrated technique for mapping out atom placements in the active site of enzymes that overcomes previous limitations in finding the positions of hydrogen atoms and reveals atomic-resolution images of the unfolding reaction.
Why is this important?
Many structural probes are not able to determine the positions of hydrogen atoms, limiting their ability to create a true picture of how molecules fit together or their chemical interactions. By bringing together the techniques of high-magnetic-field solid-state nuclear magnetic resonance, X-ray crystallography, and computational chemistry, the full atomic detail of the active chemical site of tryptophan synthase is revealed. This new chemical resolution helps unlock the potential for finding better matches for new therapeutics that target these sites.
Who did the research?
Jacob B. Holmes,1 Viktoriia Liu,1 Bethany G. Caulkins,1 Eduardo Hilario,1 Rittik K. Ghosh,1 Joana Paulino,2 Xiaoling Wang,2 Gwladys Riviere,3 Frederic Mentink-Vigier,2 Joanna R. Long,3 Michael F. Dunn,1 Leonard J. Mueller 1
1University of California – Riverside; 2National MagLab; 3University of Florida
Why did they need the MagLab?
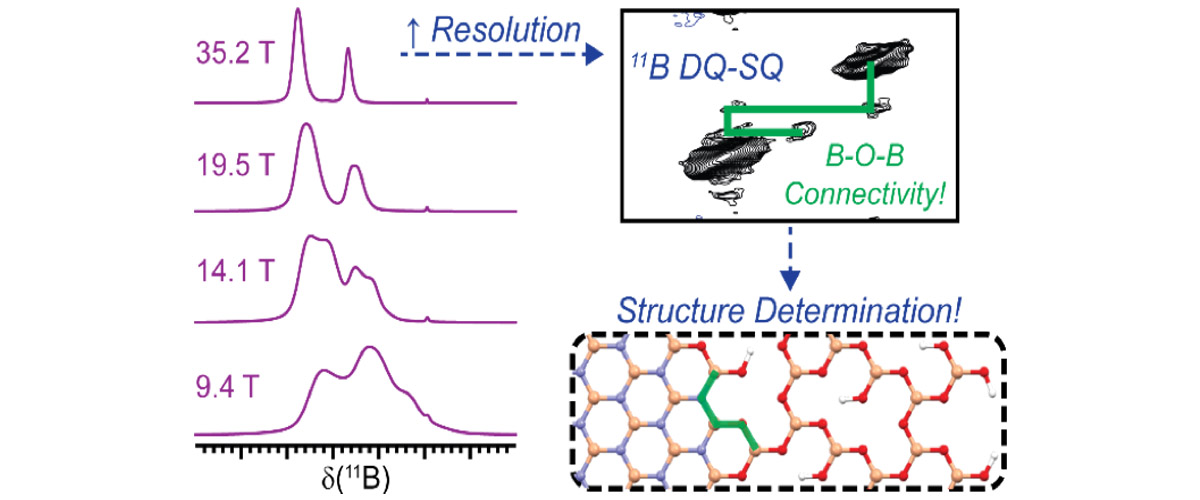
The chemical resolution of the images depends upon the strength of the magnetic field and the sensitivity of the NMR probes used to acquire the signals. The MagLab’s leading NMR probe technology development and world-record 35.2T magnetic field strength of the series connected hybrid magnet provides the sharpest possible images of the chemically-active site using NMR crystallography.
Details for scientists
- View or download the expert-level Science Highlight, Imaging Enzyme Active Site Chemistry Using Multiple Fields up to 35.2T: NMR crystallography of tryptophan synthase
- Read the full-length publication, Imaging active site chemistry and protonation states: NMR crystallography of the tryptophan synthase α-aminoacrylate intermediate, in Proceedings of the National Academy of Sciences of the USA (PNAS)
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1644779); L.J. Mueller (NSF CHE-1710671; NIH GM097569); J.R. Long (NSF CHE-1229170; NIH GM122698)
For more information, contact Robert Schurko.