What did scientists discover?
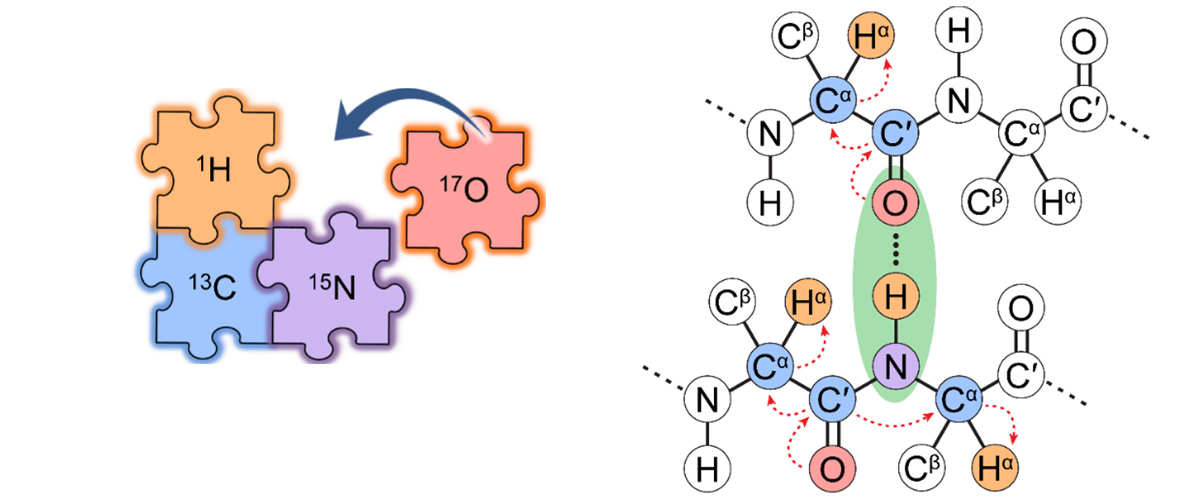
MagLab users and researchers have developed an experimental protocol and state-of-the-art nuclear magnetic resonance (NMR) instrumentation that integrates 17O into the solid-state NMR toolkit for investigating biomolecules like peptides, proteins, and enzymes. The addition of 17O represents the last "piece of the puzzle", which alongside of NMR of nuclei like 1H, 13C and 15N, enables the structural characterization of biomolecules.
Why is this important?
The elements oxygen, hydrogen, carbon, and nitrogen together form the peptide backbones of proteins. But of the four atoms comprising proteins, only H, C, and N atoms have widely been used for protein structure determination using solid-state NMR spectroscopy. The oxygen atoms have so far been the missing piece, since 17O NMR spectra have weak signals and poor resolution.
Oxygen NMR can help scientists study and understand the structural environments of oxygen atoms, including chemical bonds formed with other molecules and proteins. But oxygen has only only one NMR-active isotope, 17O, which has low natural abundance (0.037%). Furthermore, 17O has a nuclear spin of I = 5/2, meaning it is a quadrupolar nucleus (I > ½); this can result in interactions that produce very broad peaks in 17O solid-state NMR spectra. These two factors make NMR experiments on 17O much more difficult than those on more "NMR friendly" nuclei with I = 1/2, like 1H, 13C, and 15N.
This new protocol uses a special device called an ultrafast magic-angle spinning NMR probe, which was built in-house at the MagLab and allows detection of 17O indirectly via protons (1H). The strong 1H NMR signal helps to overcome the issues of poor sensitivity and low resolution, enabling the addition of 17O to the NMR toolkit for biomolecules. Special peptides enriched with 17O, 13C and 15N were necessary for this study, and produced by the MIT group using an efficient synthetic labeling method.
Who did the research?
Ivan Hung1, Eric G. Keeler2, Wenping Mao1, Peter L. Gor'kov1, Robert G. Griffin2, Zhehong Gan1
1National High Magnetic Field Laboratory; 2Massachusetts Institute of Technology.
Why did they need the MagLab?
This work uses an 18.8T magnetic field and a special magic-angle spinning NMR probe built at the MagLab that can spin the NMR sample container up to ~100kHz (i.e., it rotates 100,000 times per second). The high magnetic field and ultrafast sample spinning are critical for enhancing both the 1H NMR signal and resolution, which, in turn, is crucial for indirect 17O detection.
Details for scientists
- View or download the expert-level Science Highlight, Integration of 17O for Solid-State NMR Studies of Peptides and Proteins
- Read the full-length publication, Residue-Specific High-Resolution 17O Solid-State Nuclear Magnetic Resonance of Peptides: Multidimensional Indirect 1H Detection and Magic-Angle Spinning, in Journal of Physical Chemistry Letters
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1644779, NSF DMR-2128556); R.G. Griffin (NIH AG058504, GM132997, GM132079)
For more information, contact Robert Schurko.