What did scientists discover?
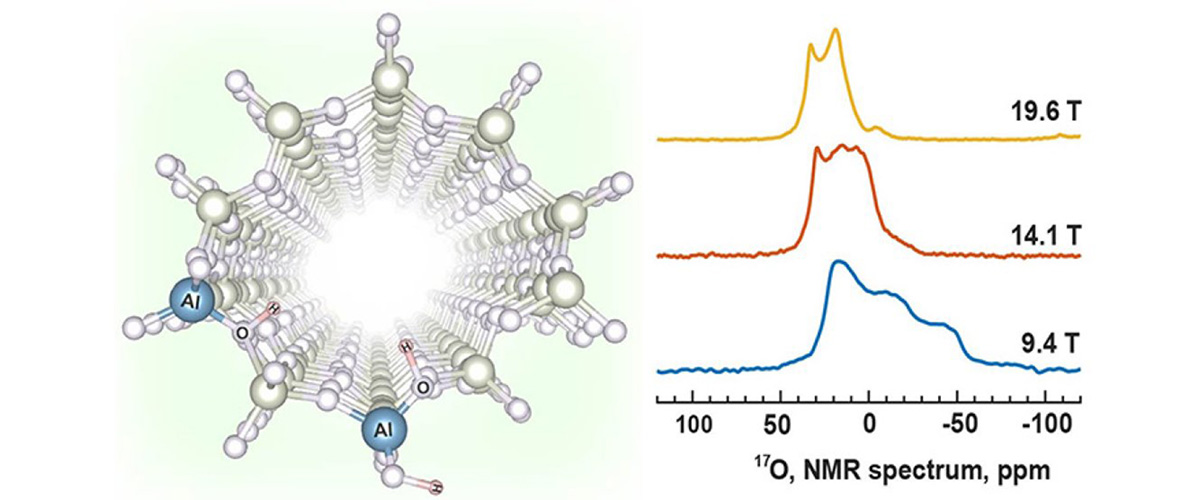
Crucial details on the structures and proximities of active catalytic sites in zeolite catalysts are revealed by ultra-high-magnetic-field solid-state nuclear magnetic resonance (ssNMR) methods that include enrichment of the catalyst by 17O isotopic substitution.
Why is this important?
Zeolite catalysts are widely used in modern industrial processes, such as petrochemical refinement and biomass conversion. Economic, environmental, and engineering requirements dictate that fundamental understanding of structure and performance drive new catalyst developments. Recent studies show that active catalytic sites are more complex than previously thought. A phenomenon of great importance is the synergistic effect, where the reactivity of two proximate (nearby) active sites is increased relative to that of two sites separated by a large distance. Ultra-high-magnetic-field 17O ssNMR experiments conducted on a variety of catalyst samples enriched with 17O isotopes revealed potential synergistic site structures. Such information is crucial for the rational design, synthesis, and manufacture of catalysts with improved efficiency and yields.
Who did the research?
Kuizhi Chen,1 Anya Zornes,2 Vy Nguyen,3 Bin Wang,3 Zhehong Gan,1 Steven P. Crossley, and Jeffery L. White2
1National MagLab; 2Oklahoma State University; 3University of Oklahoma
Why did they need the MagLab?
Ultra-high-magnetic-field (UHMF) ssNMR spectroscopy of 27Al and 17O nuclei provides structural details that cannot be obtained from experiments at lower fields, offering an unprecedented combination of high signal and high resolution that is required for these observations. This is made possible by the combination of UHMF ssNMR platforms and state-of-the-art probes available only at the MagLab.
Details for scientists
- View or download the expert-level Science Highlight, 17O Labeling Reveals Paired Active Sites in Zeolite Catalysts
- Read the full-length publication, 17O Labeling Reveals Paired Active Sites in Zeolite Catalysts, in JJournal of the American Chemical Society
Funding
This research was funded by the following grants: J. L. White (CHE-1764116 and CHE-1764130); G.S. Boebinger (NSF DMR-1644779)
For more information, contact Robert Schurko.



![Metabolism pathways of [2H7]glucose (green stars), with red dots marking the presence of 2H, and larger dots indicating two 2H atoms. Note that HDO (gold stars) can be produced at multiple steps in the glycolytic pathway.](/media/mnyndfnd/august2020_amris_cancer_metabolism.jpg)