What did scientists discover?
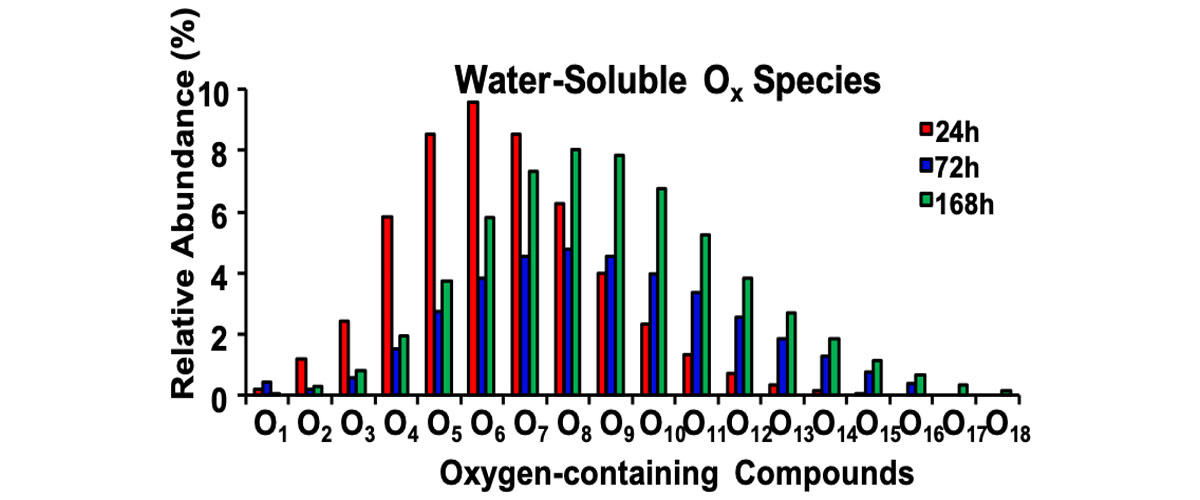
In a matter of weeks, sunlight can chemically transform marine plastics into thousands of compounds that dissolve in the ocean. The number and characteristics of these compounds differ between consumer plastic shopping bags (from Target, CVS, and Walmart) and pure polyethylene, the polymer used to make these bags. These differences are driven by chemicals added to the plastics to improve performance and appearance, as well as to lower costs.
Why is this important?
Plastic was initially believed to be inert in the environment. This work contributes quantitative information to the growing understanding that sunlight can transform plastics into highly complex mixtures of chemicals with unknown fates and impacts. While plastics research to date has largely studied pure polymers, these results show that the various consumer plastics that are actually in the environment behave differently due to additives they contain. Therefore, understanding the fates and impacts of plastic pollution - and developing next-generation materials that readily break down in the environment – will require a shift towards research that studies plastics that are more representative of those leaked into the environment.
Who did the research?
Anna N. Walsh,1,2 Christopher M. Reddy,1 Sydney F. Niles,3 Amy M. McKenna,3 Colleen M. Hansel,1 and Collin P. Ward1
1Woods Hole Oceanographic Institution; 2Massachusetts Institute of Technology; 3Florida State University
Why did they need the MagLab?
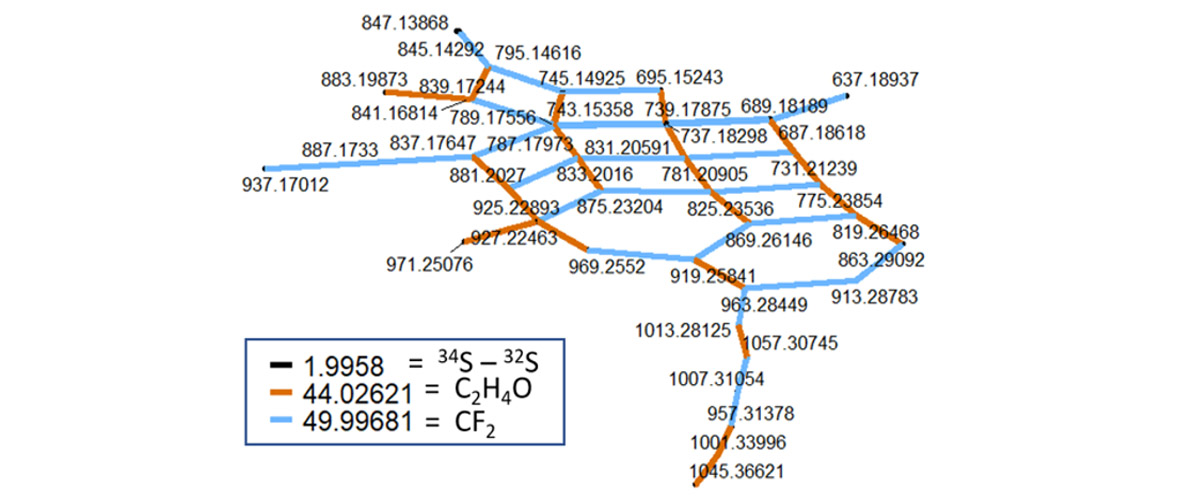
The 21 Tesla Fourier transform ion cyclotron resonance mass spectrometer offers the highest mass resolving power and mass accuracy in the world. This MagLab magnet system allowed MagLab users to measure at least ten times more sunlight-produced compounds than others had observed using less powerful mass spectrometers.
Details for scientists
- View or download the expert-level Science Highlight, Sunlight converts plastics into diverse chemical mixtures
- Read the full-length publication Plastic Formulation Is an Emerging Control of Its Photochemical Fate in the Ocean., in Environ. Sci. Technol.
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1644779); NSF Graduate Research Fellowship Program; The Seaver Institute, Gerstner Family Foundation; Woods Hole Oceanographic Institution
For more information, contact Christopher Hendrickson.