What did scientists discover?
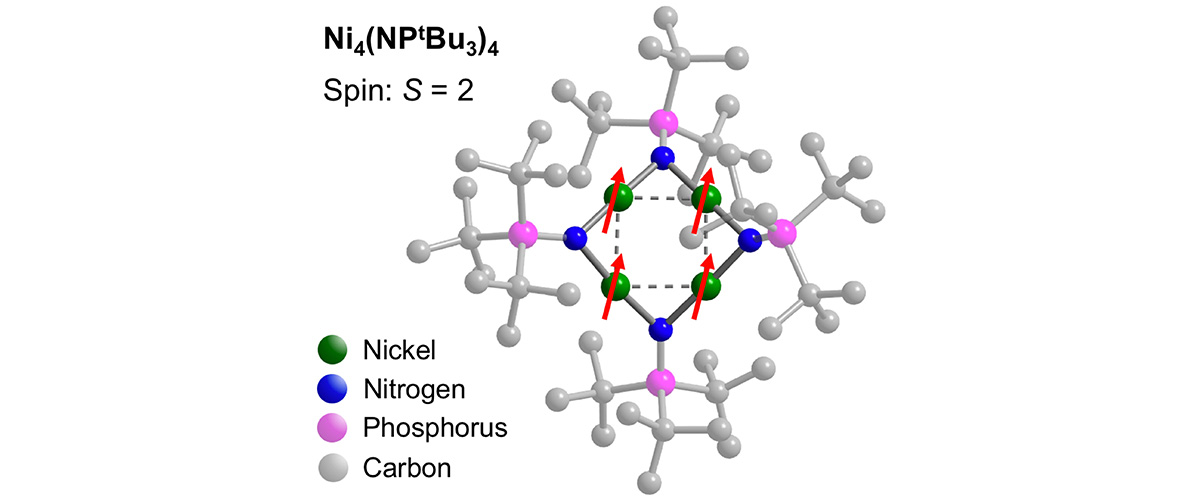
This study demonstrates the possibility of designing magnetic molecules featuring direct metal-metal bonds, similar to those found in metallic ferromagnets such as elemental iron.
Why is this important?
The work identifies new strategies for bottom-up synthetic assembly of robust ferromagnetic nanoparticles using low-cost, earth-abundant elements that may one day operate at room temperature or above. Such objects hold promise for the development of future molecular-scale magnetic storage applications, featuring information densities exceeding those of current technologies by one to two orders of magnitude.
Who did the research?
K. Chakarawet1, M. Atanasov2,3, J. Marbey4, P. C. Bunting1, F. Neese2, S. Hill4 and J. R. Long1,5
1UC Berkeley; 2Max Planck Inst. for Coal Research; 3Bulgarian Academy of Science; 4National MagLab FSU; 5LBNL
Why did they need the MagLab?
Due to the strong magnetic interactions among the itinerant electrons in these metallic nanoparticles, high magnetic fields combined with spectrometers covering a wide range of microwave frequencies are essential for gaining fundamental insights into this promising new class of magnetic materials. Such world-unique experimental capabilities can only be found within the MagLab’s Electron Magnetic Resonance (EMR) facility.
Details for scientists
- View or download the expert-level Science Highlight, Strong Magnetic Coupling in Molecular Magnets through Direct Metal-Metal Bonds
- Read the full-length publication, Strong Electronic and Magnetic Coupling in M4 (M = Ni, Cu) Clusters via Direct Orbital Interactions Between Low-Coordinate Metal Centers, in Journal of the American Chemical Society
Funding
This research was funded by the following grants: G. S. Boebinger (NSF DMR-1644779); S. Hill (NSF DMR-1610226); J. R. Long (NSF CHE-1800252)
For more information, contact Stephen Hill.