What did scientists discover?
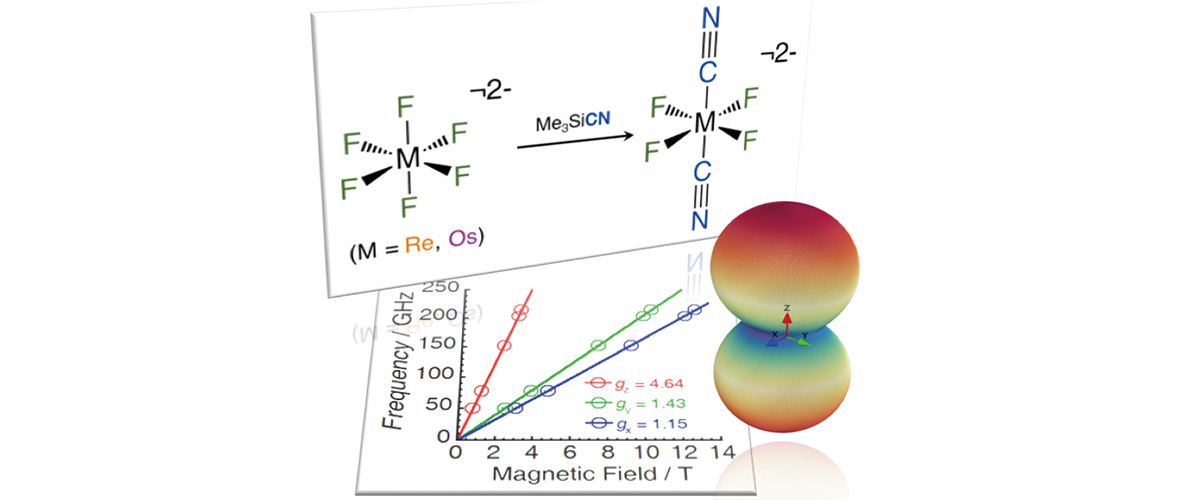
This study reports the first transition metal molecules featuring both fluorine and cyanide ligands (see "branches" attached to the metal atom (M) in the molecules at the top of the figure). A strong and significant enhancement of the non-uniformity of the magnetism, the "magnetic anisotropy" for the trans-[ReIVF4(CN)2]2- complex (shown in the upper right) was discovered by combined high-field magnetization and electron paramagnetic resonance (EPR) spectroscopy (see lower Figure).
Why is this important?
This research highlights an efficient new strategy for synthesizing molecular building blocks based on heavier transition metals that feature relatively large magnetic moments and very strong magnetic anisotropy. Such building blocks may form the basis for future high-performance magnetic materials used in high-density information storage applications.
Who did the research?
J.-L. Liu1,2,3, K.S. Pedersen1,4, S.M. Greer5, I. Oyarzabal1,6, A. Mondal1,7, S. Hill5, F. Wilhelm8, A. Rogalev8, A. Tressaud2, E. Durand2, J.R. Long9, R. Clérac1,2
1CRPP, Bordeaux; 2Univ. Bordeaux; 3Sun Yat-Sen Univ.; 4TU Denmark; 5FSU & National MagLab; 6Univ. Basque Country; 7IIS Bangalore; 8ESRF Grenoble; 9UC Berkeley & LBNL
Why did they need the MagLab?
EPR provides a direct measure of magnetic anisotropy, evidenced by the different steepness of the red, green, and blue lines in the lower figure. Magnetic anisotropy gives rise to broad EPR spectra that require very large magnetic fields in order to resolve all of the relevant details. For this reason, the measurements had to be performed at the MagLab’s Electron Magnetic Resonance facility.
Details for scientists
- View or download the expert-level Science Highlight, Molecular magnetic building blocks
- Read the full-length publication, Access to Heteroleptic Fluorido-Cyanido Complexes with a Large Magnetic Anisotropy via Fluoride Abstraction, in Angew. Chem.
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1157490, DMR-1644779); J.R. Long (NSF CHE-1800252); R. Clérac (ANR & CNRS)
For more information, contact Stephen Hill.