What did scientists discover?
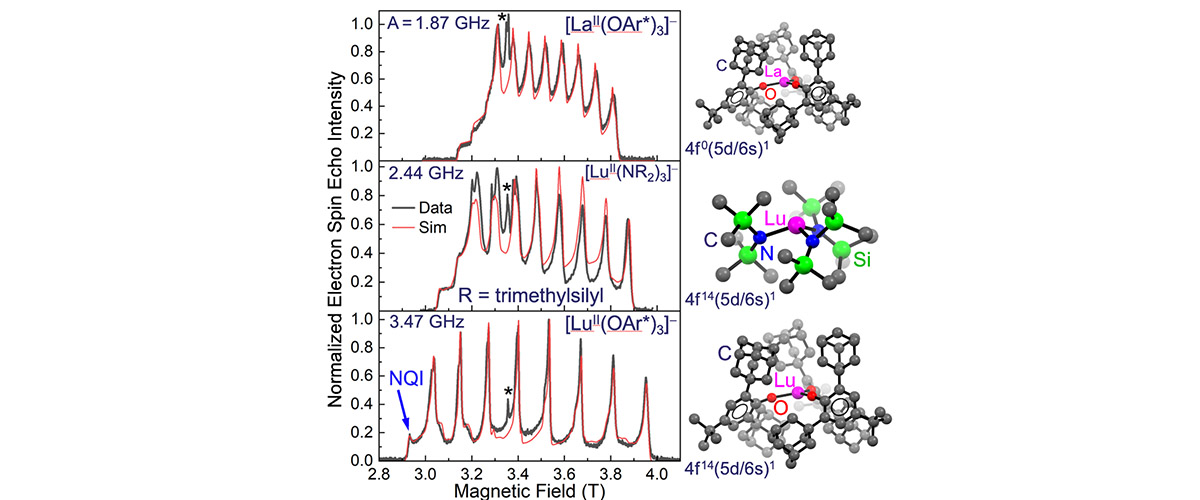
High-power pulsed electron spin-echo measurements at high magnetic fields demonstrate that it is possible to exert synthetic control over the hyperfine coupling between electron and nuclear spins in molecular lanthanide compounds. In turn, this allows chemists to enhance electron spin coherence via engineering of so-called clock transitions.
Why is this important?
The strategy of employing clock transitions to enhance quantum coherence is employed in trapped-ion quantum computers. This study shows that the same approach is viable in magnetic molecules, which show promise for next-generation quantum technologies because chemical self-assembly of magnetic molecules may eventually prove to be more scalable than trapped-ion architectures.
Who did the research?
K. Kundu1, J. R. K. White2, S. A. Moehring2, J. M. Yu2, J. W. Ziller2, F. Furche2, W. J. Evans2, S. Hill1
1National MagLab and Florida State University; 2University of California, Irvine
Why did they need the MagLab?
Studies of spin coherence require advanced spectrometers that operate in pulsed mode. The strong electron-nuclear hyperfine coupling deduced from this study gives rise to huge spectral splitting patterns that are not resolvable using commercial low-field instruments; hence the need for the HiPER spectrometer at the MagLab (Figure). Several other features of this instrument proved essential for this investigation, including (i) its uniquely high-power (1kW) that enables coherent spin manipulations on nanosecond timescales, and (ii) a rapid sample loading capability that facilitates rapid transfer of the air-sensitive samples that were synthesized under cryogenic conditions and shipped to the MagLab under liquid nitrogen.
Details for scientists
- View or download the expert-level Science Highlight, Massive Hyperfine Interaction in a Lu(II) Qubit
- Read the full-length publication, 9.2-GHz clock transition in a Lu(II) molecular spin qubit arising from a 3,467-MHz hyperfine interaction, in Nature Chemistry. Data Set
Funding
This research was funded by the following grants: G. S. Boebinger (NSF DMR-1644779); F. Furche (NSF CHE-2102568); W. J. Evans (NSF CHE-1855328); S. Hill (DOE DE-SC0020260)
For more information, contact Stephen Hill.