What did scientists discover?
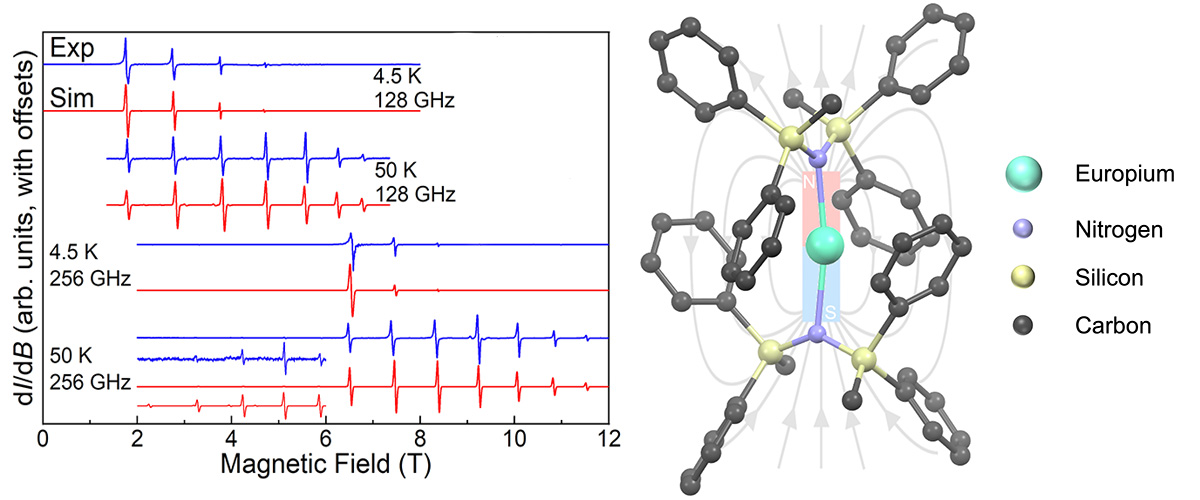
This study discusses the creation of a special magnetic molecule where two nitrogen atoms are connected to a europium ion in a nearly straight line. Unlike the usual state where europium loses three electrons, here it loses only two, making it different. The molecule can be magnetized to keep its 'North' and 'South' poles aligned even at low temperatures. High-field electron paramagnetic resonance (EPR) measurements were done to understand this behavior better and compare it with theoretical predictions.
Why is this important?
This is the first example of a europium-based single-molecule magnet (SMM), a molecule that can be permanently magnetized. This property was unexpected because europium is non-magnetic in its more-common state, however this work suggests new strategies for the development of SMMs, which have exciting potential for molecular-scale data storage applications.
Who did the research?
D. Errulat1, K. L. M. Harriman1, D. A. Gálico1, E. V. Salerno2, J. van Tol2, A. Mansikkamäki3, M. Rouzières4, S. Hill2, R. Clérac4, M. Murugesu1
1University of Ottawa, Canada; 2National MagLab (FSU), USA; 3University of Oulu, Finland; 4University of Bordeaux and CNRS, France
Why did they need the MagLab?
High-field EPR is the best way to obtain detailed microscopic information about magnetic molecules. By measuring at various microwave frequencies across a wide range of magnetic fields, researchers can hone in on the magnetic properties that effect single-molecule magnets (SMMs). The unprecedented range of magnetic fields and microwave frequencies available through the MagLab EMR facility were therefore central to this finding.
Details for scientists
- View or download the expert-level Science Highlight, Slow Relaxation of the Magnetization in a Quasi-Linear Europium(II) Molecule
- Read the full-length publication, Slow magnetic relaxation in a europium (II) complex, in Nature Communications Data Set
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-2128556); Mansikkamäki (Acad. of Finland); Hill (DOE DE-SC0020260); Clérac (CNRS & QMBx); Murugesu (NSERC)
For more information, contact Stephen Hill.