Platinum is not just for jewelry anymore. The lustrous metal and its neighbors on the periodic table are indispensable in our modern world, from your phone to your car. That’s why researchers at the National High Magnetic Field Laboratory are working to better understand how the rare elements bond with other elements to form complex molecular structures.
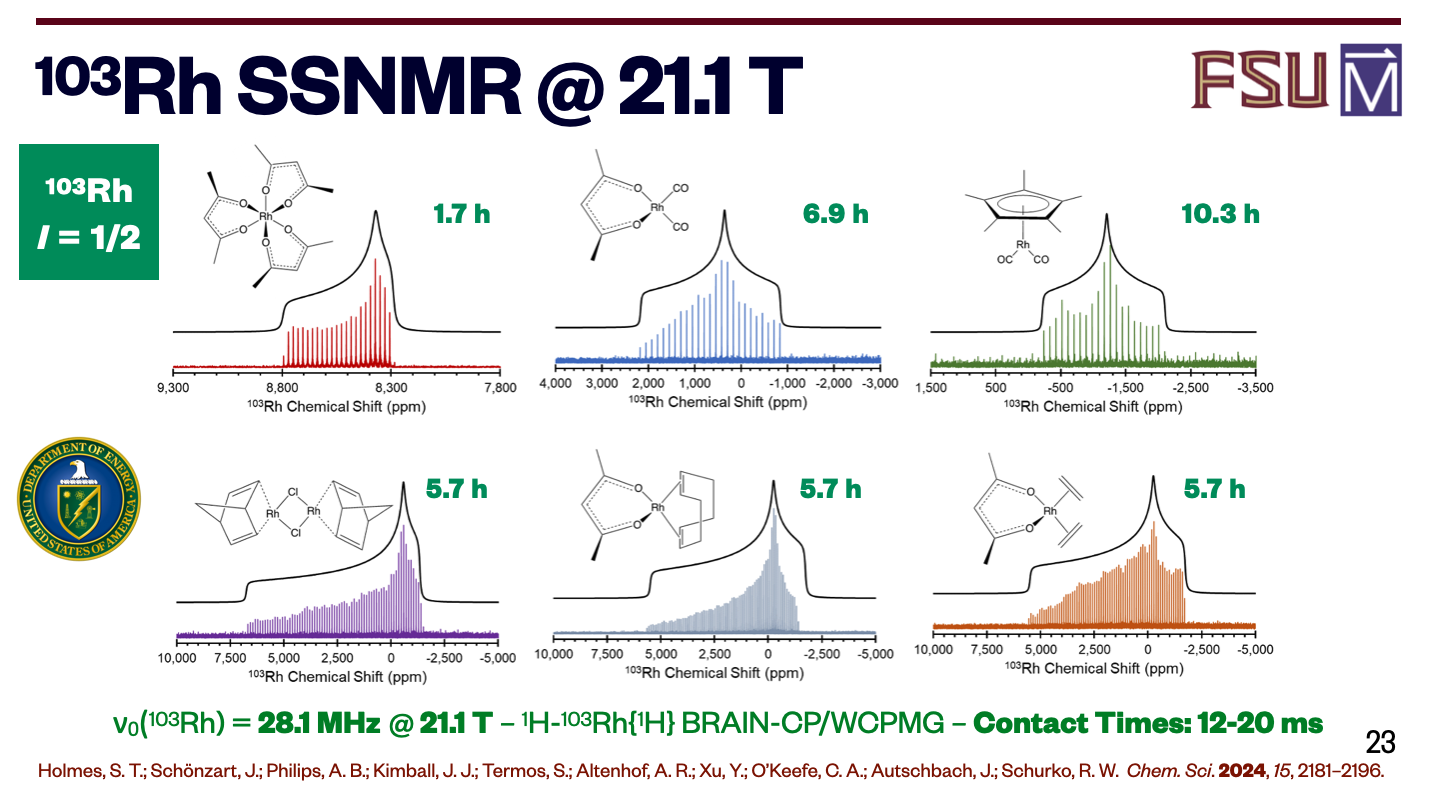
The Department of Energy is providing $1.185 million to continue the MagLab’s investigation of these precious metals using the lab’s world-leading Nuclear Magnetic Resonance systems.
“Our research helps DOE’s three-pillared strategy for addressing the need of the nation for these critical minerals and materials: diversifying supply, improving reuse and recycling, and finding replacement elements” said Rob Schurko, Director of the MagLab’s NMR program and a chemistry professor at Florida State University.
The platinum group elements, or PGEs, include their namesake and similar metals ruthenium, rhodium, palladium, osmium, and iridium. The names may be obscure, but their uses are not. PGEs are critical in dozens of applications. They’re used to refine crude oil and manufacture TV, computer, and cell phone screens. They can be found in dental fillings, medical implants, and chemotherapy drugs. And PGEs are a key component in the catalytic converters that clean your car’s exhaust.