At the MagLab, our extremely powerful magnets allow us to see, manipulate and measure the building blocks that make up our universe. Everything is made of atoms — matter that cannot be broken down into smaller units by chemical means. Understanding their structure, behavior, and interactions is essential to grasp the foundations of chemistry.
Each atom has the same atomic ingredients: protons, neutrons, and electrons. Protons have a positive electrical charge, neutrons have no charge, and electrons have a negative charge. The protons and neutrons hang out in the atom’s center, or nucleus, and the electrons zoom around it at defined energy levels. Most of the atom is made of empty space between those subatomic particles.
The basic structure of atoms can be shown with what’s called the Bohr model, where the electrons orbit the nucleus like planets orbiting the sun in our solar system.
The electron orbits, or shells, each hold a fixed number of electrons. The first shell holds up to 2. The second shell can hold up to 8, and the third shell 18. These shell amounts follow the formula 2n2, where 'n' is the number of the electron shell. At the MagLab, our powerful magnets help us understand the electronic structure of materials.
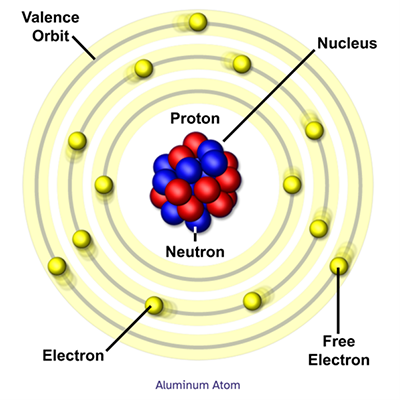
Aluminum Atom
An atom's structure is key to understanding what form an element takes and what other elements it will bond with. The electrons in the outermost shell, called valence electrons, determine how stable or reactive an element is. Atoms can lose or share their valence electrons with other elements in chemical reaction.
The 92 elements found in nature are differentiated by their atomic number, consisting of the number of protons in the nucleus, which matches the number of electrons orbiting.
They are laid out according to their atomic number in the period table of the elements.
Elements in the same column share similar characteristics, forming groups or families. Horizontal rows indicate the filling of electron shells.
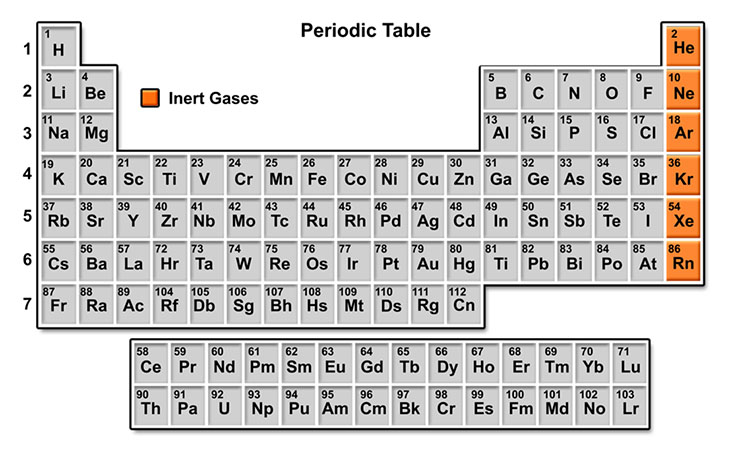
Periodic Table
The lightest element, hydrogen, is first in the periodic table, with one proton and one electron.
The heaviest element, uranium, has 92 of each.
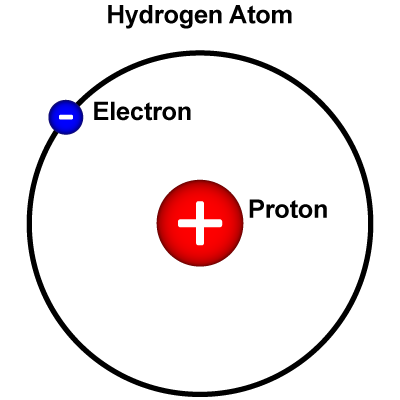
Hydrogen (H) is the simplest kind of atom. The nucleus of a hydrogen atom is made of just one proton. Around the nucleus, there is just one electron. Hydrogen is essential for life, and it is present in nearly all the molecules in living things. It occurs in vast quantities as part of the water in oceans, ice packs, rivers, lakes, and the atmosphere. As part of innumerable carbon compounds, hydrogen is present in all animal and vegetable tissue and in petroleum.
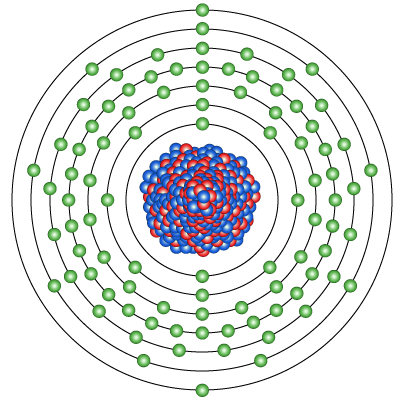
Diagram of the nuclear composition and electron configuration of an atom of uranium-238 (atomic number: 92), the most stable isotope of this element. The nucleus consists of 92 protons (red) and 146 neutrons (blue). 92 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Uranium is a radioactive element with atomic number 92 and element symbol U. This gray metal is used in ammunition, armor, nuclear weapons, and nuclear power plants.
This fun craft project demonstrates an atom’s basic structure as you create your own atomic ornament for Holiday-time or just anytime you want to celebrate science.
Make atomic ornaments for one (or all) of these four common elements:
Carbon, with an atomic number of 6, can be found in a pencil’s graphite or in a beautiful diamond. Graphene, a single atomic layer of carbon, is studied extensively at the MagLab because of its unique properties.
Nitrogen, with an atomic number of 7, makes up more than three-quarters of the Earth’s atmosphere. At the MagLab, we use liquid nitrogen, which forms below -384°, to cool some magnets and samples during research.
Lithium, with an atomic number of 3, is a soft silvery metal often used in batteries. MagLab uses its world-record MRI to image lithium and research ways to build better batteries.
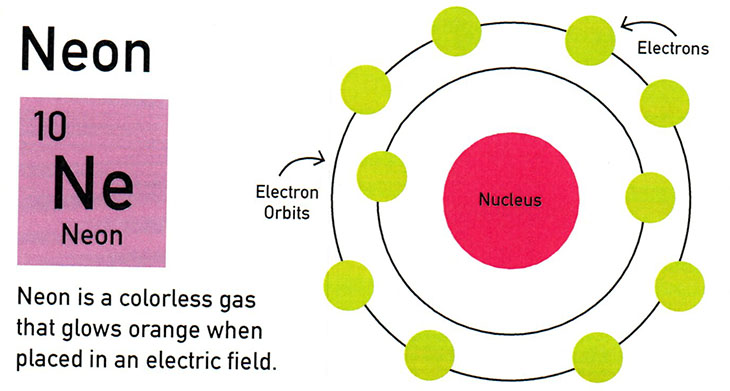
Neon, with an atomic number of 10, is a colorless gas that glows orange when placed in an electric field. It’s used for the “Open” sign you see in a shop window, or the glowing beer logo on the wall at a bar.
What you'll need:
Instructions
1. Pick an atomic element card: Lithium, Carbon, Nitrogen, or Neon.
2. The pom-pom will be the atom’s nucleus. Tie the string around the pom-pom, near the middle of the string. It doesn’t need to be exact. Do not trim the string.

3. All four atoms have two rings (inner ring and outer ring) that orbit the atom’s nucleus. Count how many electrons are on each ring. For example, Neon has two electrons for the inner ring and eight electrons for the outer ring.
4. The pipe cleaner will be the orbital rings. Cut a pipe cleaner into two pieces. One piece should be about 5 inches long, making the other roughly 7 inches.
5. The smaller piece of pipe cleaner is the inner ring. The beads will be our electrons. Put the number of electrons onto the pipe cleaner and then curve it into a circle. Repeat that process with the outer ring.

6. Place the inner ring around the nucleus (the pom-pom). Wrap the string on each side around the pipe cleaner so that the nucleus floats in the center of the ring. Repeat that process with the outer ring.

7. Tie a knot on each end to hold the rings in place.
8. Bend the paper clip into a hook.

9. Tie one end of the string to the hooked paper clip. Trim off the extra string at the bottom of the atom.

Did you know?
- The nucleus of protons and neutrons is only a tiny fraction of the overall size of the atom. If the nucleus were the size of Earth, the radius of the atom would stretch all the way to the sun.
- While only 92 elements occur in nature, scientists have produced at least 23 heavier elements in the lab using nuclear reactions. Plutonium, which has 94 electrons, is one such element.