This probe is so ingenious you might think it was whipped up by wizards. Not quite. But before you can understand what's so "magic" about this device, and why it has been a boon to scientists studying solid materials, you must first know some Nuclear Magnetic Resonance (NMR) basics.
NMR Primer
Scientists can use magnets to study the molecular composition of materials — not just what atoms they're made up of, but exactly how one atom is oriented toward the next.

Example of an NMR spectrum.
That's because atoms and the particles they're made of can be charged, negatively or positively, and can also spin like tops in different directions. So these particles are like tiny magnets themselves. Not only do they respond to each other in very particular ways based on their spin and charge, but they also respond very predictably to outside magnetic field. So when you put a material inside a certain magnetic field, as is done in NMR, its constituent particles react in ways that reveal to scientists how it is built — how far apart the atoms are and how they fit together.
When the atoms respond to specific radio waves transmitted inside the magnet, they convey data about themselves that is depicted in a spectrum, like the one above. It may not look like much to you, but to scientists the width, shape and height of each bump describes a particular atom in the molecule they're studying.

The "magic angle" is 54.7 degrees to the magnetic field.
These NMR experiments work best with liquids, because in liquids molecules are free to move around and tumble rapidly. Scientists put the molecules in a tube called a rotor and sometimes spin them around within the magnetic field. As a result, identical, unbound molecules respond to the magnet's field identically, allowing scientists to examine their collective response to the magnetic field, and thus decipher their structure.
But in solids molecules don't move much at all: Each molecule is stuck in a particular orientation to surrounding molecules as well as to the magnet. The fixed, random orientations result in fuzzy NMR signals. For years, except for the simple case of single crystals, there was no effective way to interpret the blurry spectrum from solid materials.
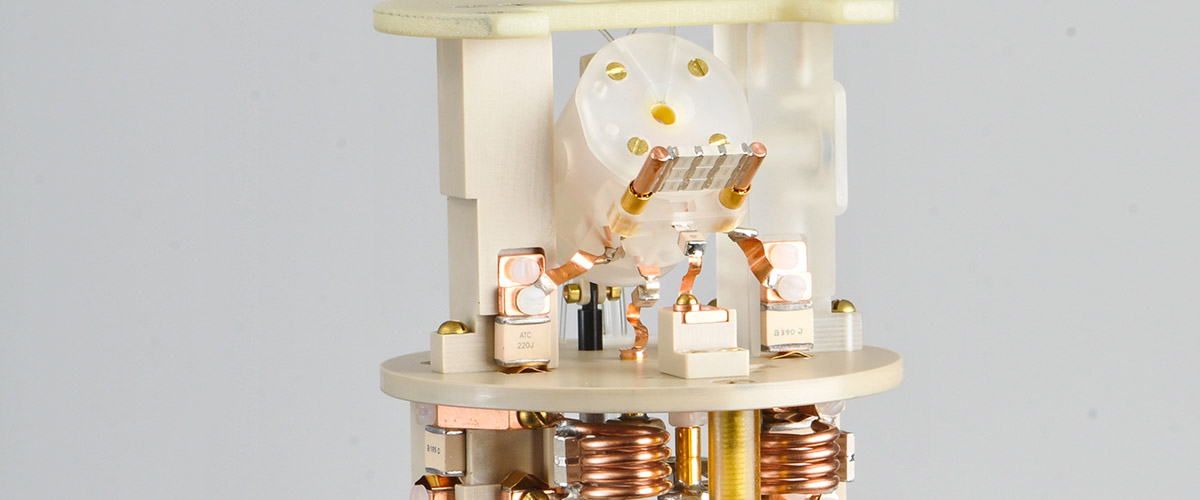
Scientists solved this problem with the wave of a big magic wand in the shape the magic angle spinning (MAS) probe. In fact, magic angle spinning was invented by the late E. Raymond Andrew, a world-renowned physicist who played a major role in establishing the vision for the MagLab during his years at the University of Florida in Gainesville, home to one of the MagLab's three branches.
Works like magic
The magic comes from a special way to, in effect, erase the influence of these neighboring molecules. If the sample, crushed into a powder form, spins within a magnetic field at a very particular angle to that field — 54.7 degrees, to be precise — the influence of a molecule's particular environment is cancelled out. (This has to do with reasons of quantum mechanics that we won't delve into here.)
For this magic trick to work, the sample must spin fast. How fast?
A circular saw spins about 100 times a second. A race car engine can spin up to 250 times per second. A typical dentist drill — one of the fastest man-made spinning objects on the planet — whirls around about 7,000 times a second.
But that's a snail's pace compared to MAS probes. The MagLab's fastest MAS probe is 10 times faster than that dentist drill, spinning 50,000 times per second. That's so fast you can barely discern a blur. It's so fast, it creates sound waves beyond your capacity to hear.
What makes this tiny tube (no more than a few millimeters across) spin so fast?

The higher the magnetic field, the clearer the data.
Nothing but air. Well, that and its clever design. Jets of air are directed at a tiny, carefully crafted turbine positioned at one end of the sample tube. This whirling turbine makes the tube spin. And because air bearings keep the tube in place, creating a narrow strip of air just a few microns wide between the bearings and the tube, there is no friction (other than that of air) to slow the tube down. A pair of optical fibers monitor and help regulate the tube's speed.
Magic angle spinning NMR has grown into a powerful tool for studying the chemistry of materials. At the MagLab, we develop fast, high-sensitivity MAS probes that fit into our special magnets, such as the world's strongest magnet, the 45 tesla hybrid. Our combination of high-quality probes, high fields and skilled user support allows us to provide visiting scientists with the highest spectral sensitivity available anywhere for many types of experiments.
Recently, we combined our low-E technology with magic angle spinning to improve the results of NMR analysis for proteins. This approach was so successful that visiting scientists asked us to build copies of the probes for their home laboratories. Finally, it was adopted by the NMR spectrometer industry.
You won't find MAS probes in any Harry Potter tome. Even so, they are examples of some very skillful science sorcery.
For more information, please contact Public Affairs.